Introduction to tDCS neurostimulation for autism
One of the treatment modalities that has shown the greatest promise for reducing symptoms of autism in recent years is transcranial direct current stimulation (tDCS). The most recent study was authored by a French group of clinicians and researchers and published in July this year [1]. This study confirmed and expanded on the findings of previous investigations, which strongly indicate that tDCS could have positive effects on cognition, behaviour and physical health, and improve quality of life and autonomy for a large percentage of individuals with autism. This study was followed by another publication describing significant improvement and in motor skills in a small group of children with autism who received tDCS treatment [2].
What is tDCS and how does it work?
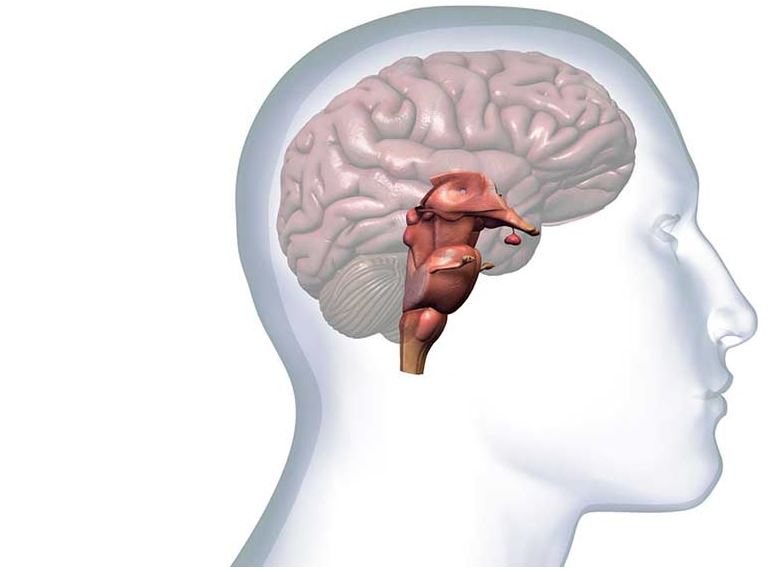
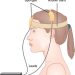
tDCS is a non-invasive brain stimulation technique in which a small hand-held device delivers a very weak electrical current to specific areas of the brain in order to promote and enhance brain plasticity. The current is delivered via electrodes connected to two sponges that are placed on the head, in correspondence to specific brain regions. The electrodes are linked to a portable device which produces a constant weak current of between 0.5–2 mA, equivalent to the strength of a 9-volt battery. Thus tDCS has a subtle effect – rather than forcing neurons into action, a small portion of the current that penetrates the scalp gently ‘nudges’ and modulates neuronal excitability.
tDCS is inexpensive, easy to apply, and has a well-established safety profile, and as such presents one of the most promising treatment modalities for a range of medical and neurodevelopmental conditions.
A brief overview of tDCS as a treatment for neurological, psychiatric & neurodevelopmental disorders
Numerous studies have highlighted the potential of tDCS for treating various neurological and psychiatric disorders, including: epilepsy, schizophrenia, ADHD, tics and obsessive compulsive disorder, migraine, motor and movement disorders (ataxia, Parkinson’s disease, dystonia, catatonia), depression, tinnitus, cerebral palsy, dementia and mild cognitive impairments, traumatic brain injury and post-stroke recovery including loss of language, social cognition and behaviours [3]–[14].)
The European Chapter of the International Federation of Clinical Neurophysiology released an article in 2017 proposing Level B recommendations (=probable efficacy) for tDCS effectiveness in depression, tinnitus addiction/cravings, and some types of chronic pain including fibromyalgia [15].
Although tDCS was originally developed to treat brain injuries and neurological disorders, a number of blind, randomized, sham-controlled, and single-session studies have demonstrated its effectiveness in improving cognitive ability, memory, executive function, mood and motivation in healthy subjects. In addition, tDCS has been found to enhance physical performance – speed, precision, motor learning, strength and endurance – in healthy subjects as well professional athletes [16]–[22].
“Recent animal and human studies have confirmed that tDCS induces significant and long-lasting neuroplastic effects, establishing the potential of this technique for therapeutic purposes”
Stagg and Nietsche 2011
A history of tDCS for autism
Small-scale studies and case reports on the efficacy of tDCS for improving functioning and behaviours in individuals with autism are numerous and span back over a decade.
In 2007 a group of Russian researchers first investigated the effects of ‘transcranial micropolarization’, a modified version of tDCS, in 17 children with autism. The treatment resulted in significantly improvements in cognitive function and language skills [23].
Those results were in line with positive results observed by other Russian investigators, in which microcurrent therapy led to improved speech and listening attention in children with developmental delay and/or speech disorders [24], [25]. Another interesting finding reported by Chutko and colleagues was that the analysis of quantitative EEG pointed to a possible correlation between positive behavioural and cognitive response to tDCS treatment and changes in alpha and theta-band activity in crucial areas of the brain.
Several years later an open label study investigated the effect of tDCS on language acquisition in a group of 10 minimally verbal children and adolescents with autism. Significant improvement in syntax acquisition and vocabulary was observed after a single 30 minute treatment session [26].
In 2013 a group of clinicians at the University of Napoli described a case of a 26 year old individual with autism and intellectual disability who had undergone several psychosocial and pharmacologic interventions to reduce his highly disabling behaviour. Those interventions were without any effect and the patient continued to manifest grossly disturbed and dangerous behaviours, including severe irritability, agitation, hyperactivity, and lack of compliance.
In consideration of the severity of the patient’s symptoms and the failure of other therapies, tDCS was administered for 10 consecutive weekday sessions. The treatment led to an overall substantial improvement in patient’s challenging behaviours and sociability: “The improvement in dysphoria, anxiety, and tantrums, conveyed by a 30% reduction in the ABC subscale for irritability, parallels previous evidence on the beneficial effect of tDCS on depression and anxiety. Of greater potential impact on rehabilitation programs for patients with AD was the impressive reduction in the ABC subscale scores for social withdrawal (45%) and for hyperactivity and lack of compliance (58%).”
The clinical improvement was still present at a 3-month follow-up visit, and no adverse effects were reported except for a slight, temporary skin irritation at the site of stimulation [27].
“We describe the first case of a patient with AD, who, after undergoing a tDCS course, displayed a dramatic reduction in his behavioral abnormalities.”
D’Urso et al. 2013
In 2014 a group of Russian investigators published their observations of positive changes after the course of tDCS in a small number patients with autism seen in their clinic. The improvements were noted in the areas of mood, speech comprehension and communication, fine motor skills and social integration (Kozhushko et al. 2014).
The first randomised controlled trial examining the effect of tDCS in the treatment of patients with autism was also published in 2014, involving 20 children with autism. Participants in the active tDCS arm of the trial experienced significant improvements in both CARS and ATEC autism scores compared to the sham tDCS arm. In addition, significant improvements were shown in two secondary measures – Children’s Global Assessment Scale and Clinical Global Impression – in the active tDCS group compared to sham. All improvements were sustained at seven days post-treatment follow-up [29].
In their follow-up study published the next year, Amatachaya and colleagues found a significant association between the EEG alpha activity and improvement in autism symptoms, confirming earlier observation of such correlation by Chutko and colleagues.
This randomized double-blind controlled placebo crossover trial involved another group of 20 children and consisted of anodal tDCS applied over the dorsolateral prefrontal cortex for 5 consecutive days. The increased brain activity near the site of stimulation was suggested by the authors to be one possible mechanism through which tDCS improves core and comorbid symptoms of autism [30].
“Our pilot study suggests that tDCS shows promise as a method to enhance working memory and prefrontal cortex function in adults with high-functioning ASD…
Remediating working memory deficits could improve some of the core cognitive and behavioral deficits that characterize ASD.”
van Steenburgh et al. 2017
Two other studies published in 2015 recorded significant and lasting improvements in several measures following tDCS treatment. In the first study 12 minimally verbal young adults with autism and intellectual disability received a 10 daily applications of tDCS. Eight out of 10 study completers improved in their abnormal behaviours, reaching an average reduction of 26.7% of the total ABC score. The most remarkable and statistically significant change was seen in the subscale assessing hyperactivity and non-compliance [31]d.
The second publication was a case study reporting lasting improvement in a 14 year old girl with ASD and drug-refractory catatonia. The patient’s rigidity, impulsivity, stereotypical behaviours and refusal to eat and to drink fully recovered after 28 sessions of tDCS. A mild improvement was observed for symptoms of grimacing, staring, negative mood, oppositional and combative behaviours. The girl progressively improved in grasp reflex and motor planning. She started eating, and by the end of the course of treatment she was able to feed herself [32].
tDCS has been shown to enhance working memory in both healthy adults and in various clinical populations, and in 2017 a pilot study by van Steenburgh and colleagues confirmed positive effects of tDCS on working memory-related task performance in 12 adults with autism [33].
Working memory (WM) refers to the capacity to maintain, update, and manipulate information held in ‘temporary storage’, and as such it is critical for many complex cognitive functions including language, general intelligence, cognitive flexibility, ability to read facial expressions, emotional regulation and breaking from restrictive or repetitive behaviours.
Although some of the other impairments in executive function in autism tend to get less pronounced with age, WM deficits usually persist into adulthood. Poor WM is found in most children and adults with autism and is thought to underlie some of the core deficits of cognition and social functioning. Improving WM function is therefore one of most promising approaches for reducing many of the difficulties experienced by individuals with autism.
“This open-label clinical study, conducted on a small sample of young adult autistic patients with highly challenging behaviours, demonstrated that tDCS can benefit patients with low-functioning AD. Indeed, it was able to reduce, in a clinically and statistically significant way, core autistic behaviours, i.e., irritability, agitation, crying, as well as social withdrawal, lethargy, hyperactivity, and non-compliance, without causing any adverse events.
Being safe, easy to apply, and economical, tDCS could have a significant role in the management of autistic symptoms for which no specific treatments currently exist.”
D’Urso et al. 2015
Three studies on tDCS were published during 2018. In the first one the authors presented a case of an 18-year old patient with autism who was experiencing lots of social difficulties, including increasing difficulty in understanding the motivations of others and understanding the rules of conversation. The patient also had a history of depression and anxiety, unresponsive to psychotherapy.
The treatment, consisting of 30min of tDCS sessions applied for 8 consecutive days, led to a substantial improvement in the patient’s social functioning, which was maintained at 2 months and 1 year follow-ups. This included increased interest in social interactions and engaging in spontaneous, on-topic conversations, increased awareness of others’ feelings and perspectives, improved ability to stay on topic and reduced interruptions during conversations. The patient also reported lessened feelings of anger and frustration over social disappointments [34].
The same team of researchers published the results of investigations into the effectiveness of tDCS paired with social skills treatment in six adults with autism and normal intellectual ability. Participants scored significantly higher marks in verbal fluency after receiving active treatment compared to sham tDCS. The emotion verbal fluency part of the test, specifically, indicated significant differences when comparing real to sham tDCS conditions [35].
Also in 2018 a double blind randomised trial involving 26 children was carried out by a team of Chinese investigators. Each patient received 10 tDCS treatments over the dorsolateral prefrontal cortex once every two days. The results showed that the treatment effectively altered the excitatory and inhibitory imbalance and induced increases in EEG complexity, specific to the site of stimulation. This led the authors to suggest that EEG complexity at the stimulation site could be used as an outcome measure in clinical trials [36].
Finally, a study published in July of 2019 looked into possibility that tDCS could improve executive dysfunction in patients with high-functioning autism. The treatment involved eight patients who received ten consecutive 15 minute sessions of tDCS sessions. This study showed significant improvement in initiation, cognitive flexibility and repetitive and restrictive behaviours [1].
Mechanisms of action of tDCS with relevance to autism
Modulation of brain function – enhancement of neuronal plasticity by tDCS
Neuroplasticity, or brain plasticity, is the term used to describe the ability of the brain to change continuously throughout an individual’s life. A healthy brain reacts and adapts to changing environmental demands by remodelling itself – by changing its structure, function and ‘neuronal wiring’ (the way neurons communicate with each other). Such changes need to happen on an ongoing basis in order to for the brain to be able to meet the changing demands and challenges from the environment, and to regain function after an insult or injury.
Brain plasticity is of major importance for normal brain function, cognition and behaviour. Pathological changes in neuroplasticity of the brain, or in other words inability of the brain to adapt according to changing demands, plays an important role in neurological and psychiatric disorders, including autism.
tDCS has been shown to improve ability of both healthy as well as neurologically impaired individuals in various domains of cognition and executive function. By exciting brain regions involved in working memory and attention, researchers and clinicians have reported achieving significant improvements in domains of working memory, processing speed, arithmetic processing, behaviour control (especially impulse inhibition and frustration tolerance), multi-tasking, attention shifting and many others [37]–[45].
Numerous studies have investigated the effects of tDCS, and the mechanisms through which it enhances brain plasticity and signalling ability of neurons. The primary effect of tDCS is generally believed to involve modulation of resting membrane potential, or electrical charge or neurons (neurons being in effect ‘electrical devices’).
“If the brain map could normalize its structure in response to abnormal input, the prevailing view that we are born with a hardwired system had to be wrong. The brain had to be plastic.”
M. M. Merzenich
The membrane potential of neurons determines how much calcium and other signalling molecules are trafficked in and out of the cell. This in turn influences connectivity and communication between neurons, neuronal energy metabolism, gene expression, production and release of other signalling molecules such as GABA, serotonin, dopamine, adrenaline, endocannabinoids and others [46]–[52].
Since all types of cells and tissue that are present in the brain and the nervous system are sensitive to electric fields, the therapeutic action of tDCS in the brain might extend beyond its modulation of neuronal excitability. Brain glial cells as well as endothelial and other cells that form brain blood vessels, are likely modified by tDCS, since it has been observed to modulate both brain inflammation as well as brain blood perfusion [53]–[56].
In short, tDCS is capable of influencing varied pathological processes and pathogenic cascades in the central nervous system.
tDCS stimulates language-related brain areas and modulates auditory processing
A growing body of research shows that tDCS is capable of modulating linguistic abilities in healthy individuals as well as patients who suffer aphasia, or loss of language. In healthy adults a single-session of tDCS has been shown to have significant effects across all language measures, sometimes even after a single session. The reported improvements in non-verbal patients were in some cases remarkable [57]–[59].
Changes in language comprehension and speech are thought to be partially due to tDCS-induced changes in brain excitation and neuroplasticity in the areas of the brain that process language, better connectivity between different parts of the brain, as well as general improvements in cognition or working memory.
Another mechanism behind tDCS-related improvements in language and communication abilities is likely connected to auditory sensory processing. tDCS has been shown to affect the capacity to selectively focus on a particular speaker of interest in a complex acoustic environment [60]–[63].
This ability of tDCS treatment to improve filtering of speech from background noise could be particularly relevant for individuals with autism, since poor ‘speech discrimination’ and difficulties in encoding of speech at the brainstem are well-documented features of autism [64]–[68].
tDCS for epilepsy and subclinical seizures in autism
The prevalence of seizure disorders in autism is significantly higher than in the general population and epilepsy is the leading cause of premature death in individuals with autism. Furthermore, subclinical epileptiform activity has been found in a large majority of individuals with autism, even in the absence of a clinical seizure disorder.
Epilepsy and autism likely share common pathogenesis. Individuals with autism and epilepsy are more likely to have severe social impairments and life-long dependency than those diagnosed solely with autism. Autism often occurs in epilepsy-associated syndromes such as Landau-Kleffner syndrome, Dravet Syndrome, and tuberous sclerosis complex.
Growing evidence highlights the potential of tDCS and other forms of neurostimulation for drug-resistant, or refractory, epilepsy. Experimental animal studies demonstrate that tDCS could reduce the number and intensity of seizures, and early human trials indicate that it could be helpful for at least a subgroup of patients [69]–[70].
One of the earliest studies on humans was conducted at the Russian Academy of Medical Sciences and published in 2001. Transcranial micropolarization, a form of tDCS, was administered to 18 children with drug resistant epilepsy, of whom 13 had cerebral palsy and five had organic CNS lesions. All children experienced a significant and lasting reduction in the frequency and intensity of seizures, paralleled by clear improvements in EEG parameters [71].
In the West, encouraging results have been reported by preliminary single-session safety studies conducted at the Max-Planck-Institute, Harvard Medical School and others (Assenza et al. 2017; Baudewig et al. 2001; Fregni et al. 2018; Lin et al. 2018; Siebner et al. 2004; Tecchio et al. 2018; Zoghi et al. 2016).
A group of researchers at the Khon Kaen University Thailand was one of the first to explore the feasibility of cathodal tDCS for pediatric seizures. In their study a single 20 minute session resulted in a significant reduction in epileptiform discharges up to 2 days after application. The treatment was well-tolerated by all 36 children who took part [79].
The same team also studied the effects of tDCS on twenty-two patients with Lennox-Gastaut syndrome, a severe refractory epileptic syndrome. Five consecutive days of cathodal tDCS applied over the primary motor cortex reduced seizure frequency and epileptic discharges, with the effects sustained at 3 weeks follow up investigation [80].
“We found a significant association between improvements in the ATEC social and health and behavioral problems subscale and an increase in peak alpha frequency in those who received active tDCS treatment.
The findings demonstrating an increase in (cortical activity) near the site of stimulation provide preliminary evidence for a possible mechanism of the effects of tDCS on a measure previously found to be associated with autistic severity in individuals with ASD.”
Amatachaya et al. 2015
A study of 10 adult patients with refractory epilepsy conducted by Karvigh and colleagues and published in 2017 looked at the effects of High-Definition-tDCS, which uses a higher number of electrodes than the standard tDCS. The improvements in epileptiform discharges and seizure frequency were not statistically significant for the whole group. However, the patients experienced significant improvements in attention and working memory and this effect was sustained one month after the treatment [81].
In the most recently published studies by Yang and colleagues seven patients with epileptic spasms, a type of severe drug-resistant seizures, received a total of eighteen 14-day blocks of cathodal tDCS treatment. Three of seven patients experienced sustained seizure reduction. Treatment was well tolerated in all patients [82].
Their second study, published in September 2019, was a randomized, double-blind trial involving 82 patient with refractory focal epilepsy. Fourteen consecutive days of tDCS significantly decreased seizure frequencies in patients in active treatment compared to sham stimulation. The protocol using 2 × 20-min daily stimulation was superior to the protocol using 20-min daily stimulation only [83].
In addition to pre-designed trials, several case studies have reported positive and sometimes dramatic effects of tDCS for patients with refractory epilepsy. For example in the report by San-Juan and colleagues a patient with cortical dysplasia and refractory epilepsy, characterized by 10-15 daily right tonic hemi-body seizures, received seven sessions of cathodal tDCS. This resulted in a lasting reduction of seizures frequency to one single seizure per month, limited to patient’s upper arm. Another case report of two patients with refractory Continuous Spike-Wave Discharges During Slow Sleep showed a large reduction of interictal epileptiform EEG discharges after only three sessions of cathodal tDCS [84]–[87].
tDCS modulates physiological response to stress
Individuals with autism have exaggerated response to stress. They tend to overreact to perceived threat and are unable to handle uncertainty. The sympathetic ‘fight or flight’ stress response is longer lasting in autism compared to healthy population.
This over-responsivity to stress correlates with deficiencies in adaptive functioning and worsens many of the additional problems frequently present in individuals with autism, such as anxiety, avoidance of novel situations and rigid and/or challenging behaviours including irritability and aggression.
Elevated stress response and dysregulation of the HPA axis in response to stress, as found in individuals with autism, can potentially predispose to a number of mental and physical health disorders. The rates of many medical disorders are significantly increased in autism, as are the rates of anxiety, major depression and suicide.
tDCS has been observed to modulate the physiological response to stress. Data from multiple studies show that tDCS sessions can successfully reduce potentially harmful increases in cortisol levels and heart rate in both healthy individuals as well as patients with anxiety disorders [88]–[91].
In addition to mitigating negative consequences of stress on the physical health, one study has found that tDCS also reduces stress-induced working memory deficits, pointing to a ‘potential new avenue to prevent stress-induced cognitive impairments [92].
Furthermore, tDCS significantly reduces pathological fear and modulates the fear memory in experimental conditions, indicating that it could provide a useful treatment modality for anxiety, post-traumatic stress disorder and other disorders of emotional regulation (Herrmann et al. 2016, 2018).
Chronic stress is associated with hyperalgesia, or increased pain sensitivity. Individuals with heightened or prolonged stress response often have lowered pain threshold, which is caused by stress-related changes in neural pain pathways. Data suggests that treatment with tDCS could prevent stress-induced pain sensitation [45], [73].
Effects of tDCS on sensory dysfunction and pain processing
Individuals with autism often present with sensory dysfunction and abnormal pain sensitivity. Both children and adults with autism suffer increased rates of both chronic and acute pain compared to typical population. The mechanisms of increased pain sensation in autism are thought to be at least partially related to abnormal processing of pain signals by the brain and the nervous system [95]–[98].
It is thought that chronic pain may reflect not only local inflammation but have origins in the central nervous system reflecting maladaptive changes in brain excitability.
Most of the studies so far demonstrate that tDCS can reduce the pain originating from disturbed pain processing signals by the peripheral and central nervous systems. Good results have been obtained in trials on tDCS for chronic back pain and fibromyalgia, and it has even been effective in reducing chronic abdominal pain in patients with inflammatory bowel disease, unrelated to inflammation and disease activity [99], [100].
As most of the studies so far employed anodal current stimulation over primary motor cortex area of the brain, it has been suggested that the mechanisms behind the pain relieving effects of tDCS may include the regulation of synaptic connections and brain sensory pathways, as well as changes in the levels of brain neurotransmitters linked to processing of pain, such as glutamate, dopamine, serotonin and endogenous opioids [101]–[103].
As discussed in previous chapter, tDCS can also reverse some of the detrimental effects of chronic stress on pain sensitivity, and decrease levels of brain pro-inflammatory molecules, including TNFα (see the following chapter) [104].
Effects of tDCS on motor learning and motor dysfunction
Many people with autism experience substantial motor difficulties. Deficits in fine and gross motor skills in young children with autism are strong predictors of expressive language development and severity of autism symptoms and impairments in later life. In addition to generally poor motor skills, various disorders of movement control are very common in autism, including ataxia, akinesia, dyskinesia, tic disorders/Tourette syndrome and catatonia.
“The survival of a biological entity depends on its ability to accurately control the motion of its limbs, its head, and its eyes. Our nervous system provides us with the ability to learn this control, and the ability to maintain calibrated and accurate movements…
Remarkably, this form of motor adaptation in humans can be readily up-regulated or down-regulated by non-invasive stimulation of either the motor cortex or the cerebellum.”
Orban de Xivry et al. 2015
Multiple studies have found that tDCS improves acquisition of motor skills when applied during learning of motor tasks, and that it enhances general motor function such as speed and accuracy of movement. The improvement in motor function is even more marked for fine motor skills compared to gross skills.
These motor-enhancing effects of tDCS have been observed in healthy individuals, including children, as well as neurologically impaired patients. Those effects are accompanied by notable changes in neuronal activity and brain connectivity [105]–[109].
Of possible great relevance to autism are the preliminary findings published in 2019 that strongly tDCS could improve speech and motor learning. The results of the small single-session study by Buchwald and colleagues indicated that the improvements might be greater when tDCS is applied immediately before motor-speech learning practice, compared to applying it during a learning session [110].
The combination of brain stimulation and motor exercises shows promise for improving motor performance in children with autism, as demonstrated by recently published research by Mahmoodifar and Sotoodeh. 18 children with autism received motor skills training alongside either active or sham tDCS. The children in the active tDCS group achieved significantly higher improvements in motor control and balance [2].
“The combination of brain stimulation and motor exercises seems to show particular promise for improving motor performance in children with ASD”
Mahmoodifar & Sotoodeh 2019
With regards to movement disorders emerging evidence points to the ability of tDCS to restore brain inhibition pathways and improve some of the symptoms in a wide range of conditions including ataxia, cerebral palsy, Parkinson’s disease, tremor, dystonia, and catatonia [75], [111]–[117].
In addition to modifying neuronal excitability and instigating the formation of new brain connectivity pathways, tDCS also leads to changes in the levels of some neurotransmitters. Several studies in animal models show tDCS modulating brain dopaminergic pathways providing another putative mechanism for its positive effects in Parkinson’s disease and tic disorders [5], [118]–[120].
“Cerebellar modulation can improve specific symptoms in some movement disorders and is a safe and well-tolerated procedure”
Franca et al. 2018
Effects of tDCS on inflammation
tDCS and other forms of neuromodulation have long been suspected to have effects that extend far beyond the brain and the nervous system. Numerous animal and in vivo studies show that tDCS influences inflammatory and immune responses in the central and peripheral nervous systems, such as for example the gut enteric nervous system [50], [121]–[124].
Translational clinical trials of those findings are still limited. tDCS has shown promising results for reducing pain in patients with osteoarthritis, and one recently published study demonstrated that active tDCS, compared to placebo sham treatment, reduces levels of proinflammatory molecules IL-6, IL-10, TNF-a in patients with painful arthritis. These findings suggest that treatment with active tDCS may have additional therapeutic benefits in patients with pro-inflammatory conditions, beyond reducing their pain sensitivity [125]–[127].
In addition to classic neuroinflammatory diseases, such as multiple sclerosis, inflammation and skewed immune reactions are implicated in autism and in most neurological and psychiatric conditions. Hence, through its possible influence on the inflammatory response, tDCS may constitute a promising therapeutic option for such disorders.
tDCS – remaining questions and practical considerations
Is tDCS painful?
Depending on external and internal factors such as electrode placement, level of current, and length of the tDCS session, feelings may differ between people. Commonly reported sensations include itching, warming and tingling feelings at the source of the electrodes, which can usually be reduced through application of saline solution. Despite these minor sensations, tDCS is generally a positive experience that does not inflict any pain or feelings of discomfort.
Is tDCS safe?
So far, there is no evidence of long-term side-effects or injury caused by tCDS. Information from controlled trials involving over 20,000 patients show that side-effects are minor and are solely restricted to the location of the electrode on the scalp. These include temporary itching, tingling, and redness of the skin A Antal et al. 2017; Palm et al. 2016).
Evidence from experimental animal models indicates that brain injury by Direct Current Stimulation could only occur at current densities that are over an order of magnitude above those produced by conventional tDCS (Bikson et al. 2016; Jackson et al. 2017).
“To date, the use of conventional tDCS protocols in human trials has not produced any reports of a Serious Adverse Effect or irreversible injury across over 33,200 sessions and 1000 subjects with repeated sessions. This includes a wide variety of subjects, including persons from potentially vulnerable populations.”
Bikson et al. 2016
Regarding tDCS use in children, a study published in 2015 reviewed data from 48 studies involving more than 513 children and adolescents. The study indicated that the side-effects of tDCS and other neuromodulatory techniques were, in general, mild and transient. Most of the side effects reported specifically in tDCS pediatric trials listed: tingling (11.5%), itching (5.8%), redness (4.7%), and scalp discomfort (3.1%) [132]. Preliminary research suggests that tDCS may be well tolerated and safe for children and adolescents with psychiatric and neurodevelopmental disorders.
Is tDCS similar to electroshock therapy?
Electroshock therapy, also called electroconvulsive therapy (ECT), delivers a powerful jolt of electrical current to the prefrontal cortex of the brain. It was developed in the 1930s to treat severe cases of mood disorders. Although ECT has significant side effects, such as memory loss and cognitive impairments, it still in use today due to the fact that it can often successfully treat cases of severe depression that are unresponsive to other treatments. tDCS is in no way similar to ECT.
Moving forward – questions remaining to be answered
Studies of tDCS have so far shown large variability in its effects amongst individuals. It is unclear at this point how much of the difference is down to study design, specific tDCS montages used in the studies, or individual underlying differences in study subjects.
Further studies regarding the correct attachment locations of electrodes, tDCS stimulation intensity, duration, and frequency are needed.
Other factors to be taken into consideration, that can influence the outcome of tDCS application, include for example baseline level of neuronal activity in each individual, or difference in skull or even hair density that determine how much current penetrates the skull (Krause and Kadosh 2014; Russell et al. 2013, 2014)
Ongoing and future tDCS research for autism
At the time of writing this article several clinical trials of tDCS for treating autism are currently taking place or awaiting publication:
Effects of Transcranial Direct Current Stimulation for Enhancing Cognitive Function in Children With Autism Spectrum Disorder
Description: randomised controlled trial involving 90 participants (children)
Conducted by: The Hong Kong Polytechnic University
Details: clinicaltrials.gov/ct2/show/NCT03814083
The Use of Transcranial Direct Current Stimulation (tDCS) to Improve Communicative Efforts, Speech, Language and Related Cognitive Functions in Individuals With Autism
Description: A prospective observational trial involving 40 adult participants (adults)
Conducted by: Johns Hopkins University
Details: clinicaltrials.gov/ct2/show/NCT01603225
Transcranial Direct Current Stimulation for Cognitive Rehabilitation of Children With Autistic Spectrum Disorders
Description: randomised controlled trial involving 16 participants (children)
Conducted by: Federal University of Paraíba
Details: clinicaltrials.gov/ct2/show/study/NCT03947086
Learning Enhancement Through Neurostimulation in Autism
Description: randomised controlled trial involving 25 participants (adolescents)
Conducted by: Medical University of South Carolina
Details: clinicaltrials.gov/ct2/show/NCT02998684
The information contained in this article in no way constitutes medical advice. If you wish to consider tDCS as a treatment option please consult a qualified practitioner
References:
[1] M. Rothärmel et al., “A Prospective Open-Label Pilot Study of Transcranial Direct Current Stimulation in High-Functioning Autistic Patients with a Dysexecutive Syndrome,” Neuropsychobiology, 2019.
[2] E. Mahmoodifar and M. S. Sotoodeh, “Combined Transcranial Direct Current Stimulation and Selective Motor Training Enhances Balance in Children With Autism Spectrum Disorder,” Percept. Mot. Skills, 2019.
[3] J. Brunelin, M. Mondino, R. Bation, U. Palm, M. Saoud, and E. Poulet, “Transcranial Direct Current Stimulation for Obsessive-Compulsive Disorder: A Systematic Review.,” Brain Sci., vol. 8, no. 2, p. 37, Feb. 2018.
[4] A. R. Brunoni et al., “Transcranial direct current stimulation for acute major depressive episodes: Meta-analysis of individual patient data,” Br. J. Psychiatry, vol. 208, no. 6, pp. 522–531, Jun. 2016.
[5] V. Eapen, R. Baker, A. Walter, V. Raghupathy, J. J. Wehrman, and P. F. Sowman, “The Role of Transcranial Direct Current Stimulation (tDCS) in Tourette Syndrome: A Review and Preliminary Findings.,” Brain Sci., vol. 7, no. 12, p. 161, Dec. 2017.
[6] R. Ferrucci, T. Bocci, F. Cortese, F. Ruggiero, and A. Priori, “Cerebellar transcranial direct current stimulation in neurological disease,” Cerebellum & Ataxias, vol. 3, no. 1, Dec. 2016.
[7] A. Finisguerra, R. Borgatti, and C. Urgesi, “Non-invasive Brain Stimulation for the Rehabilitation of Children and Adolescents With Neurodevelopmental Disorders: A Systematic Review.,” Front. Psychol., vol. 10, p. 135, 2019.
[8] M.-F. Kuo, P.-S. Chen, and M. A. Nitsche, “The application of tDCS for the treatment of psychiatric diseases.,” Int. Rev. Psychiatry, vol. 29, no. 2, pp. 146–167, Mar. 2017.
[9] V. Nejati, M. A. Salehinejad, M. A. Nitsche, A. Najian, and A.-H. Javadi, “Transcranial Direct Current Stimulation Improves Executive Dysfunctions in ADHD: Implications for Inhibitory Control, Interference Control, Working Memory, and Cognitive Flexibility,” J. Atten. Disord., p. 108705471773061, Sep. 2017.
[10] A. L. Zaninotto et al., “Transcranial direct current stimulation (tDCS) effects on traumatic brain injury (TBI) recovery: A systematic review.,” Dement. Neuropsychol., vol. 13, no. 2, pp. 172–179, Jun. 2019.
[11] M. A. Salehinejad, M. Wischnewski, V. Nejati, C. M. Vicario, and M. A. Nitsche, “Transcranial direct current stimulation in attention-deficit hyperactivity disorder: A meta-analysis of neuropsychological deficits,” PLoS One, vol. 14, no. 4, p. e0215095, Apr. 2019.
[12] E. Fileccia et al., “Effects on cognition of 20-day anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex in patients affected by mild cognitive impairment: a case-control study.,” Neurol. Sci., vol. 40, no. 9, pp. 1865–1872, Sep. 2019.
[13] X.-J. Shou et al., “A Volumetric and Functional Connectivity MRI Study of Brain Arginine-Vasopressin Pathways in Autistic Children,” Neurosci. Bull., vol. 33, no. 2, pp. 130–142, Apr. 2017.
[14] T. Yuan, A. Yadollahpour, J. Salgado-Ramírez, D. Robles-Camarillo, and R. Ortega-Palacios, “Transcranial direct current stimulation for the treatment of tinnitus: a review of clinical trials and mechanisms of action.,” BMC Neurosci., vol. 19, no. 1, p. 66, Oct. 2018.
[15] J. P. Lefaucheur et al., “Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS),” Clinical Neurophysiology, vol. 128, no. 1. Elsevier Ireland Ltd, pp. 56–92, 01-Jan-2017.
[16] L. Angius, A. Pascual-Leone, and E. Santarnecchi, “Brain stimulation and physical performance,” in Progress in Brain Research, vol. 240, Elsevier B.V., 2018, pp. 317–339.
[17] X. Liu et al., “Increased interhemispheric synchrony underlying the improved athletic performance of rowing athletes by transcranial direct current stimulation,” Brain Imaging Behav., vol. 13, no. 5, pp. 1324–1332, Oct. 2019.
[18] J. Dedoncker, A. R. Brunoni, C. Baeken, and M.-A. Vanderhasselt, “The effect of the interval-between-sessions on prefrontal transcranial direct current stimulation (tDCS) on cognitive outcomes: a systematic review and meta-analysis.,” J. Neural Transm., vol. 123, no. 10, pp. 1159–72, Oct. 2016.
[19] A.-M. Kamali, Z. K. Saadi, S.-S. Yahyavi, A. Zarifkar, H. Aligholi, and M. Nami, “Transcranial direct current stimulation to enhance athletic performance outcome in experienced bodybuilders,” PLoS One, vol. 14, no. 8, p. e0220363, Aug. 2019.
[20] A. H. Okano et al., “Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise,” Br. J. Sports Med., vol. 49, no. 18, pp. 1213–1218, Sep. 2015.
[21] P. A. Pope, J. W. Brenton, and R. C. Miall, “Task-Specific Facilitation of Cognition by Anodal Transcranial Direct Current Stimulation of the Prefrontal Cortex.,” Cereb. Cortex, vol. 25, no. 11, pp. 4551–8, Nov. 2015.
[22] A. Wiegand, A. Sommer, V. Nieratschker, and C. Plewnia, “Improvement of cognitive control and stabilization of affect by prefrontal transcranial direct current stimulation (tDCS).,” vol. 9, no. 1, p. 6797, 2019.
[23] N. I. Kozhushko, V. M. Shaĭtor, E. A. Ponomareva, and N. F. Berezhnaia, “[Transcranial micropolarization in the complex therapy of early child autism.].,” Zhurnal Nevrol. i psikhiatrii Im. S.S. Korsakova, vol. 107, no. 10, pp. 47–51, 2007.
[24] V. A. Ilyukhina, N. Y. Kozhushko, Y. K. Matveev, E. A. Ponomareva, E. M. Chernysheva, and M. A. Shaptilei, “Transcranial micropolarization in the combined therapy of speech and general psychomotor retardation in children of late preschool age.,” Neurosci. Behav. Physiol., vol. 35, no. 9, pp. 969–76, Nov. 2005.
[25] L. S. Chutko, A. M. Livinskaia, I. S. Nikishena, E. A. Iakovenko, S. I. Surushkina, and A. V Sergeev, “[Transcranial micropolarization in the treatment of specific speech disorders in children].,” Vopr. Kurortol. Fizioter. Lech. Fiz. Kult., no. 6, pp. 42–3.
[26] H. D. Schneider and J. P. Hopp, “The use of the bilingual aphasia test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism,” Clin. Linguist. Phonetics, vol. 25, no. 6–7, pp. 640–654, Jun. 2011.
[27] G. D’Urso et al., Transcranial direct current stimulation for autistic disorder., vol. 76, no. 5. Elsevier USA, 2014, pp. e5-6.
[28] N. I. Kozhushko, I. D. Kropotov, I. K. Matveev, V. I. Semivolos, E. P. Tereshchenko, and A. I. Holiavin, “[Brain structures and functional pecularities in children with mental disorders and transcranial direct current stimulation].,” Fiziol. Cheloveka, vol. 40, no. 4, pp. 36–43, 2014.
[29] A. Amatachaya et al., “Effect of anodal transcranial direct current stimulation on autism: A randomized double-blind crossover trial,” Behav. Neurol., vol. 2014, 2014.
[30] A. Amatachaya et al., “The short-term effects of transcranial direct current stimulation on electroencephalography in children with autism: a randomized crossover controlled trial.,” vol. 2015, p. 928631, 2015.
[31] G. D’Urso et al., “Transcranial direct current stimulation for hyperactivity and noncompliance in autistic disorder,” World J. Biol. Psychiatry, vol. 16, no. 5, pp. 361–366, Aug. 2015.
[32] F. Costanzo et al., “Transcranial direct current stimulation treatment in an adolescent with autism and drug-resistant catatonia,” Brain Stimulation, vol. 8, no. 6. Elsevier Inc., pp. 1233–1235, 01-Nov-2015.
[33] J. J. Van Steenburgh, M. Varvaris, D. J. Schretlen, T. D. Vannorsdall, and B. Gordon, “Balanced bifrontal transcranial direct current stimulation enhances working memory in adults with high-functioning autism: A sham-controlled crossover study,” Mol. Autism, vol. 8, no. 1, Jul. 2017.
[34] J. Esse Wilson, D. K. Quinn, J. K. Wilson, C. M. Garcia, and C. D. Tesche, “Transcranial Direct Current Stimulation to the Right Temporoparietal Junction for Social Functioning in Autism Spectrum Disorder,” J. ECT, p. 1, Aug. 2017.
[35] J. Esse Wilson, M. C. Trumbo, J. K. Wilson, and C. D. Tesche, “Transcranial direct current stimulation (tDCS) over right temporoparietal junction (rTPJ) for social cognition and social skills in adults with autism spectrum disorder (ASD).,” J. Neural Transm., vol. 125, no. 12, pp. 1857–1866, Dec. 2018.
[36] J. Kang et al., “Transcranial direct current stimulation (tDCS) can modulate EEG complexity of children with autism spectrum disorder,” Front. Neurosci., vol. 12, no. APR, Apr. 2018.
[37] D. J. Fehring, R. Illipparampil, N. Acevedo, S. Jaberzadeh, P. B. Fitzgerald, and F. A. Mansouri, “Interaction of task-related learning and transcranial direct current stimulation of the prefrontal cortex in modulating executive functions.,” Neuropsychologia, vol. 131, pp. 148–159, Aug. 2019.
[38] M. A. Friehs and C. Frings, “Pimping inhibition: Anodal tDCS enhances stop-signal reaction time.,” J. Exp. Psychol. Hum. Percept. Perform., vol. 44, no. 12, pp. 1933–1945, Dec. 2018.
[39] W.-Y. Hsu, T. P. Zanto, J. A. Anguera, Y.-Y. Lin, and A. Gazzaley, “Delayed enhancement of multitasking performance: Effects of anodal transcranial direct current stimulation on the prefrontal cortex.,” Cortex., vol. 69, pp. 175–85, Aug. 2015.








 Рояна Гулузаде родилась 2 сентября 1980 года. С 1998 по 2004 год учился на педиатрическом факультете Азербайджанского медицинского университета. Аттестационная комиссия Министерства здравоохранения Азербайджанской Республики получила Сертификат неврологической аттестации.
Рояна Гулузаде родилась 2 сентября 1980 года. С 1998 по 2004 год учился на педиатрическом факультете Азербайджанского медицинского университета. Аттестационная комиссия Министерства здравоохранения Азербайджанской Республики получила Сертификат неврологической аттестации.